Introduction
According to the Competence Centre on Technology Transfer of the European Commission:
“Technology transfer (TT) refers to the process of conveying results stemming from scientific and technological research to the marketplace and to wider society, along with associated skills and procedures, and is as such an intrinsic part of the technological innovation process.”
Image 1: the stages of a generic Technology Transfer process
This generic definition covers all industries and doesn’t convey the progressive discovery stages of pharmaceuticals. Still, knowledge is a crucial concept for having a TT.
All pharma companies, of course, always had a formal or informal process to transfer their productions from R&D to pilot scale and industrial scale manufacturing. Still, for a long time, there was no clear regulatory driver. An ISPE Good Engineering guide, published in 2003, kicked off the conversation for a more formal and visible TT process , and in 2008 the International Council for Harmonisation (ICH) was the first regulator to mention TT formally:
“The goal of technology transfer activities is to transfer product and process knowledge between development and manufacturing, and within or between manufacturing sites to achieve product realisation. This knowledge forms the basis for the manufacturing process, control strategy, process validation and ongoing continual improvement” (ICH Q10, section 3.1.2) .
Current Regulation
In 2011, the WHO Technical Report 961 Annex 7 turned the spotlight on TT, defining it as:
“A logical procedure that controls the transfer of any process together with its documentation and professional expertise between development and manufacture or between manufacture sites”. It is a systematic procedure that is followed in order to pass the documented knowledge and experience gained during development and or commercialization to an appropriate, responsible, and authorized party. Technology transfer embodies both the transfer of documentation and the demonstrated ability of the receiving unit (RU) to effectively perform the critical elements of the transferred technology to the satisfaction of all parties and any applicable regulatory bodies.
After this high-profile endorsement, TT became a focus point of all regulatory audits!
There are various practical implications associated with the above. Still, in practice, pharma companies now need to define TTs and identify when a TT is required as part of their change control procedures. The TT collects and collates several documents, such as regulatory files, specifications, analytical methods, analytical method validation reports, bill of materials, process validation reports, and manufacturing procedures. The procedure should therefore determine the specific list of documents and activities required in each case. This can be tricky as concurrent changes (scale-up, technology switch, packaging change) must be managed as part of the TT activities, and similar grey areas can be problematic. TT documentation will be often and thoroughly audited, and poor TT documentation can significantly slow down time to market and increase cost, for example. On the one hand, there is a temptation to go over the top with documentation and, on the other hand, a risk of not documenting enough, hence the need to accurately specify the documents required.
Product Lifecycle and Tech Transfer
During the lifecycle of a pharmaceutical product, there can be several TTs.
After the pre-clinical studies, when phase 1 of clinical studies is initiated, a small amount of that molecule is required for safety studies, and this is often produced by an independent company specialised in this kind of supply. This, of course, requires a TT. Decisions made at this stage may have a long-term impact on the future of that product. Similarly, in phase 2 of the clinical studies, another amount of product will be required, still in small quantity in general terms, but at a higher volume than another organisation with another TT may better address.
When phase 3 of the clinical trials is often initiated, the “asset” is transferred to another organisation, better suited for finalising the more extensive clinical study. The TT that enables that is often critical for the product's long-term success. Of course, in phase 3, the volumes and dose form refinement of the clinical trial material are at another scale (for example, in pilot plants), requiring another organisation to deal with it and another TT.
It is not uncommon that the transfer of a new product from R&D / clinical trials organisations to a full-scale manufacturing site is initiated 3 to 5 years before the expected completion of phase 3 clinical studies to allow for the construction and validation of a dedicated facility for the new product introduction and production of initial stability batches. This TT will allow the product to finally reach the patient if the phase 3 clinical trial is successful and the product can be launched.
Sometime after the launch, the required volumes of that product will become more predictable, and they will be (typically) at a different level compared to the initial early-stage assessment, probably driving another TT for having a capacity adjustment.
The product will become generic a few years later, likely requiring another TT.
Several years later, when perhaps new products better addressing the same condition will be launched, the production will probably have a capacity reduction as part of the end of the lifecycle of the product. This will drive another TT.
Finally, the product will be discontinued or may be maintained only for a few patients in the world who have shown adverse reactions to alternative medication. Even at this final stage, a duty of care drives ongoing stability on the stockpiled material. Some organisations or parts of organisations are focused on the tail of the product lifecycle, which will prompt another TT.
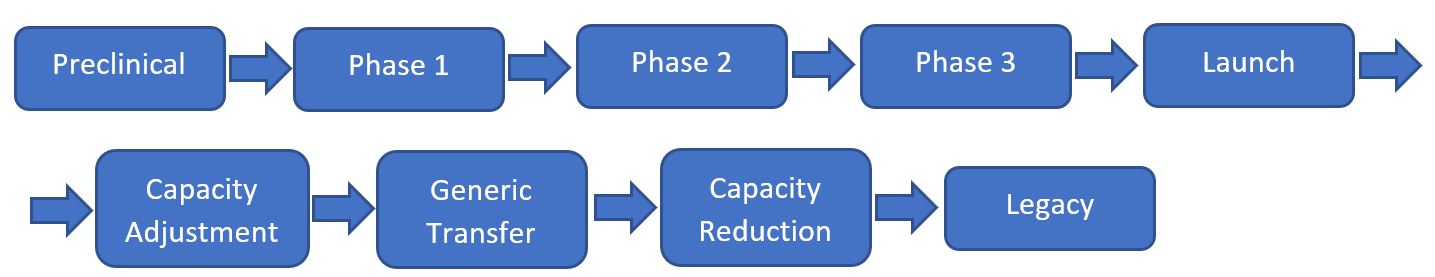
Image 2: flow chart showing an example of the lifecycle of a medicinal product, with each stage potentially needing a Tech Transfer
Practical Considerations and Examples
While it is undeniable that the spike of attention for TT after 2011 was due to the new regulatory requirements, arguably, the TT should be incorporated into the core business process of all pharmaceutical companies, as it makes business sense. It focuses on the risks and on the knowledge required to manage them. It enables collaboration and involvement (organisational alignment). It is a legacy for the future (by managing the knowledge to deliver more efficient product evolution in the future, for example).
The common issue is that current regulations don’t provide much further detail beyond the mentioned definitions and principles for a process that can have massive variations depending on the specific applications. In some way, the regulatory authorities are recognising the level of complexity and variety, but they infer: “Do it as you want, as long as you do it right!” The experience from multiple regulatory audits from multiple auditors worldwide indicates that the focus isn’t on the specific detail about how the TT has been executed but on how the knowledge has been managed within the particular context.
Ultimately, the one obvious thing is that we deal with two organisations (the Sending Unit and the Receiving Unit) that will have, in general, some similarities and some differences in their manufacturing capabilities, equipment, procedures, and training, and the TT is there to bridge those differences and allow the Receiving Unit to produce the same product and have the same positive impact on the patient that the Sending Unit has. The clear indications from the Regulators are that the guideline to overcome the discontinuity is the know-how: this is ultimately what TTs are about.

Image 3: basic principles of Technology Transfer
For example, suppose we want to do a TT from an early-stage discovery R&D organisation that discovered a promising molecule (Sending Unit) to a Contract Manufacturing Organisation (CMO) that will produce the material for clinical trials (Receiving Unit). In that case, the TT actions should focus on legal aspects / IP, characterisation, analytical methods, dosing options, and safety aspects, which are critical at this stage of development, and not on yield, cycle time, unit cost, artwork, packaging matrices, cost of disposing of unused product, that at this stage of development are not a priority.
A TT between a late-stage R&D department of a big company that is waiting for some phase 3 trial results (Sending Unit) and a manufacturing site in the same company that will execute the new product introduction to the market (Receiving Unit) will be driven by very different priorities, such as regulatory aspects, analytical methods, time to market, manufacturing scale flexibility, packaging and labelling definition, safety aspects, contingency funding, but aspects like unit cost, yield, cost of disposing of validation batches will not be that prominent in the bigger picture. Of course, while the general drive to benefit the patient will be the same as in the previous case, the action list will prioritise different activities.
The R&D department of a generics manufacturing company developing a product for the “six months exclusivity” (Sending Unit) doing a TT to a manufacturing site of the same company that will manufacture that product (Receiving Unit) will address the actions that ensure good planning, regulatory, efficiency, cost, documentation, packaging definition, scale definition. In contrast, the cost of disposing of trial batches of material will probably not be a priority.
In the case of a big pharma manufacturing site willing to outsource a well-established generic production (Sending Unit) to a CMO manufacturing site that will manufacture that product (Receiving Unit), there will probably be lots of workaround site audits, commercial and legal, continuity of supply, yield, efficiency, analytical methods, labeling and artwork, logistics, process validation, being able to release for sale as much process validation material as possible, suppliers and perhaps not so much around IP, manufacturing scale flexibility, dosing options.
A TT between the R&D department of a branded generics manufacturer (Sending Unit) that developed a variation of an existing product line and a manufacturing site of the same company already producing the same range and planning to incorporate the variations (Receiving Unit) will probably be about marketing and speed to market, labeling, and artwork transition control, efficient process validation, manufacturing scale flexibility, continuity of supply, being able to release for sale as much process validation material as possible, logistics, regulatory, rather than commercial, IP, site audit.
Let's consider the TT between one area of a manufacturing site with a product of high technical complexity (Sending Unit) and another area of the same manufacturing site, planning to manufacture the same product at different scales or different technologies (Receiving Unit). The action list will deal preferentially with regulatory, validation risk assessment and contingency planning, continuity of supply, and implicit knowledge transfer, and there will probably be no activity on analytical method validation, IP, labeling, artwork, or site audit.
The table below shows typical priorities for Tech Transfer at different stages of the product lifecycle.
|
Product Lifecycle Stage |
Legal / IP aspects |
Cost of trial batch |
Cost of product |
Packaging / Artwork |
Speed |
|
Pre-clinical |
High focus |
Practically irrelevant |
Irrelevant |
No focus |
Relevant |
|
Clinical |
Very high focus |
Practically irrelevant |
Irrelevant |
Basic requirements only |
Critical |
|
Pilot Plant |
Very high focus |
Nice to have |
Practically irrelevant |
No focus |
Critical |
|
Launch |
Very high focus |
Nice to have |
Nice to have |
High focus |
Critical |
|
Capacity Adjustment |
Not critical for internal transfers |
Important |
Important |
Not critical for internal transfers |
Depends on market demand |
|
Generic Transfer |
Critical for the “six months exclusivity”, then less critical |
Very important |
Critical |
Very high focus |
Critical for the “six months exclusivity”, then less critical |
|
Capacity Reduction |
Lower focus |
Important |
Nice to have |
Not critical for internal transfers |
Nice to have |
|
Legacy |
Lower focus |
Irrelevant |
Irrelevant |
Focus on basics only |
Lower focus |
In practice, depending on the specific circumstance, the same action may be critical, nice to have, or completely irrelevant. Aspects that can significantly impact a TT are typically linked to transfer scope, inherent complexity, project changes, stage in the product lifecycle, maturity of process technology, maturity of receiving site, cultural distance, and contractual arrangements.
Different Approaches
Another relevant aspect that can significantly differentiate TT activities is the different procedural approaches to TT in different companies. There are, in fact, two diametrically opposite approaches to TT execution:
- Procedure driven: the procedure calls for a fixed list of documents to be produced for all TTs within a specific organisation; the procedure will be reviewed and updated periodically;
- Risk-based: “suitable” Subject Matter Experts (SMEs) get together, assess the risks and, depending on the specific risk profile, generate and drive appropriate activities to manage all risks.
Neither approach is perfect, and, in reality, TT is usually a compromise between them.
The procedure-driven approach is generally more suited to relatively low-experienced executing teams/organisations with somewhat repeatable activities, engaging with skilled professionals to draft TT procedures. It requires an excellent process drafted by highly trained professionals. Still, it is resource heavy as some actions on the list will address risks irrelevant to the specific project, especially if the kind of projects varies significantly. Moreover, when the procedure fails, usually new actions are added to the procedure as CAPA, making it more and more resource demanding. This approach often struggles to address implicit knowledge requirements.
The risk-based approach is resource efficient, aligning resources with risks and allowing faster delivery within the risk management frame. However, it requires an outstanding and streetwise SME team, effectively covering all relevant aspects of the project; otherwise, it can generate disasters. It also requires continued focus from the SMEs at all stages (risk assessments are “live” documents). Generally, this approach fits mature organisations with a high emphasis on delivery times and costs, comfortable with assessing and taking risks.
Case Studies
The following are some examples of how TTs can go very wrong due to issues that the TT overlooked at the time.
A few decades ago, when microcrystalline cellulose (MCC) had recently been introduced to the market, it was discovered that an API with delicate stability in the air could be stabilised by co-formulating with MCC. At the time, only one MCC made and particle size was available on the market, so the development chemist put in his lab book and paperwork the product's commercial name instead of the chemical name. The particle size wasn’t very fitting for the API size, but there
was no other option then. Somehow, this information was transferred to the license, approved in over 100 countries with a brand name instead of a chemical denomination for the MCC component. Now there is a ubiquitous and very popular API that is stuck with a specific brand of MCC (driving higher costs than necessary, as alternative brands can’t be considered as procurement options) with a specific particle size that isn’t aligned with the particle size of the API, with recurring demixing issues (driving deviations, supply delays, re-work and, ultimately, more extra costs). The company marketing that API has decided that applying for license changes in over 100 countries, with associated fees and managing the interim situation with some countries accepting the transition faster and others 5+ years later due to slower internal procedures, is not a viable option. When the TT from the clinical scale to the industrial scale happened, the company followed a procedure-driven approach to TT, with all stages happening in silos. No one from procurement or full-scale manufacturing was involved at the pre-clinical stages to check the documentation produced and passed on to regulatory to produce the license.
About twenty years ago, an API was scaled up to the industrial scale with an intercontinental product transfer happening simultaneously (missing some intermediate pilot plant batch steps to be faster to market). Still, the trial batches provided frequent batch failures (one in four or more). Due to speed to market pressures, the deviations were never fully resolved, and the product was launched with the internal agreement that the process would be improved later on. The regulators somehow accepted technical justifications and licensed the product. The company decided to disband the team that followed this scale-up and re-assigned the various individuals to other projects. In time, the required process improvements were broadly identified, but it took years as there was no formal commitment and no dedicated team. After initial success, the product had failing fortunes (partly due to more effective cures being discovered for the same indications, partly due to the high costs driven by the high batch failure rate), and the associated revenue diminished steadily. By the time it was clear how to progress in sorting out the technical problems, the required investment wasn’t worth it anymore. The company involved, at the time, followed a procedure driven process for TT, with different departments and sites operating in silos and competing with each other.
A few years ago, a branded generic chewable tablet was given a new lemon flavour besides the original peppermint. The unique formulation did very well at the pilot plant scale. However, at industrial scale production, the tablets had a failure rate of>30% in the tableting machine, derailing the new product launch. The lemon flavour was a powder encapsulated lemon oil that fine powder coated the binding surfaces of tablet granules. This had no observable effect on the slow press in development, but in the high-speed process at an industrial scale, the bonding weakness destroyed the tablets. Nobody saw this coming during the risk assessment. The company at the time followed a risk based approach for TTs, but the change was deemed so low-risk that the risk assessment team didn’t have the right resources, i.e., the level of expertise required to identify this kind of possible issues early on.
A few years ago, a well-established dispersion was proposed for a scale-up to reduce costs and increase supply speed. Still, the license prescribed a specific agitation speed in revolutions per minute in a critical process step. That speed worked well at the current batch size but didn’t produce the required dispersion in bigger mixers, as the increased tangential speed of the mixing blades in the bigger vessel destroyed that specific dispersion. It is beneficial to communicate in the license non-dimensional numbers linked to physical states and not control parameters that will work only for a specific batch size: for example, ranges of Reynolds numbers rather than revolutions per minute, as the varying diameter of the vessels used for potential, future scale up may make the revolutions per minute parameter meaningless. In this case, some “stricter” regulatory authorities accepted with no problem adding the control parameter of having Reynolds number ranges in the license but did not accept the contextual removal of the “quality critical” parameter of revolutions per minute. Of course, the company did not proceed with the change. At the time, the TT was procedure driven in that company, and no one was involved in checking and assessing these potential issues.
Quite recently, a company transferred a branded generic suspension from one site on one continent to another newly acquired site to launch that product in another group of markets on that new continent. The TT went well, and the validation batches were used for the launch, but the follow up productions were always out of spec (sometimes high, sometimes low) for a specific preservative. After a very long investigation, it turned out that the powder preservative had the generic Ph. Eur. particle size specifications on the license. Still, in-house particle size specifications required an extra milling step and an extra cost (and this was communicated as part of the risk-based TT). To show better cost effectiveness, the new site did the validation batches with the preservative with in-house specs (as it was free shipped from the old site), but then the local site procurement acquired the standard particle size for routine production, thinking that the preservative would dissolve making the particle size meaningless. The preservative was also formulated to be partially in suspension, and the test method was designed for that specific particle size, which eventually drove the out-of-spec batches. The new site wanted to impress corporate management by cutting that corner and decided not to involve the old site in the risk assessment of this change. The TT procedure was not applied according to its spirit on this occasion.
Conclusion
During the organization's lifecycle and depending on the specific requirements of the Sending Units and Receiving Units, the most appropriate approach to TT may differ, and the knowledge gaps in that organisations may be different. This is not a reason for the patient to be worried, as from time to time, the most appropriate external specialist support can be cost-effectively hired to address the typical failures. It is crucial, however, that the organisations involved in TT are self-aware: they need to know their capabilities and need to make a conscious decision about their approach to TT procedures (that should fit as much as possible the current lifecycle of the organisation) and about addressing the potential gaps in their knowledge and personnel resources.
by Alessandro Viola, Senior Process Engineering Specialist at IPS-Integrated Project Services Limited.
This article originally appeared in Pharmaceutical Engineering.



