Expansions and renovations to existing biological facilities, and construction of new facilities, provide a unique opportunity to rethink basic design strategies and use new technologies to build a better facility that will improve compliance. This article is the sixth in a six-part series on how single-use systems (SUS) are changing the modern biotechnology facility design and construction paradigm.
As discussed in previous articles in this series, newly designed biotech facilities are profoundly different from the legacy facilities, including those built as recently as five years ago. New building material technologies, the ability to clone building sections into fixed modules, and the reduced footprint of SUS facilities provides a new portfolio of construction options. This article will discuss the emerging trends in facility construction, the available alternatives, the “dos and don’ts,” and some guidance for the future.
The State of Bioprocessing Facility Construction
The smaller footprint of single-use facilities and the mobile nature of single-use equipment enable facilities to be constructed differently than in the past. SUS has led to lower ceiling heights, consolidated process operations, and simplified implementation of single-floor processing, which has, in turn allowed these facilities to be built within warehouse-like shells, reducing cost and speeding build time. The portability of single-use systems allows for the facility core to be built on an independent timeline from the equipment, reducing overall construction duration. The continuing drive to build facilities more quickly means that parallel design, construction, and procurement activities are becoming the norm.
Specific Construction Approaches & Considerations
During the site selection process, consider the constructability and compatibility with your existing operational model, in conjunction with the typical cost and schedule considerations. This will help identify areas of concern with each of the different building approaches — grassroots, expansion, or renovation — along with the technology options. In the end, the schedule will drive the technology choice. The sections immediately below will discuss the building options, and then we will proceed to an exploration of the technology options.
Grassroots Build-Out
Building a completely new facility is an attractive approach in that you are not constrained to an existing model. However, a grassroots build can face challenges if there is not a communicated, unified vision for the facility. The key is a plant master plan that covers both the facility and the technologies it will contain. Cost concerns (which we will discuss further in a moment) often become the most contentious aspect of grassroots build-outs. The facility master plan must be specific regarding the technology direction and the user requirements for facility delivery.
Onsite Expansion
The construction of a new building on-site faces fewer constraints and existing conditions than renovations (see below) but can allow for existing operations/practices, utilities, spatial relationships, and technologies to be leveraged to minimize investment in infrastructure. If the existing plant capabilities can’t be leveraged, then a new modular/podular approach is warranted.
Building Renovations
Renovations of existing facilities require you to work around ongoing operations. This typically lends to a phased approach to construction. Segregation and timing are critical to the success of such projects. Building around the existing operations is always a risky proposition, and construction isolation must be developed using a risk-based approach, with the approval of all stakeholders, especially quality assurance (QA) and regulatory affairs.
Construction Technology Models
There are a variety of choices available for the construction of a bioprocess facility core. Each provides a unique mix of initial capital outlay, speed, and durability benefits.
Stick-Built
The traditional method of building with studs and polymer wallboard continues to be the most cost-effective solution in the short-term. However, durability and time-to-construct concerns have swung many projects to consider modular panels. Stick-built renovations can be quickly executed in the $400 to $600 per square foot range, assuming the scope is small and well defined.
Modular
Modular panels have become the mainstay of biological facility construction. They provide a durable finish and quick implementation. The wall panels often contain built-in piping, electrical, and instrumentation networks, and are connectable — functioning similarly to pipe chases. These systems also utilize walkable ceiling panels, which typically negate the cost advantages traditional studs and wall boards. The modular aspect focuses primarily on the construction of the rooms/suites. The HVAC is built within a frame structure overhead, with preplanned/precut supply and return registers. Modular built-outs (new or renovation) typically cost between $500 and $800 per square foot. Modular construction can shorten the typical 18-month construction cycle by three to six months, because the modular panels and HVAC sections are manufactured while the foundations, slab, and roof are being built in the field. The figure below shows a modular build-out.
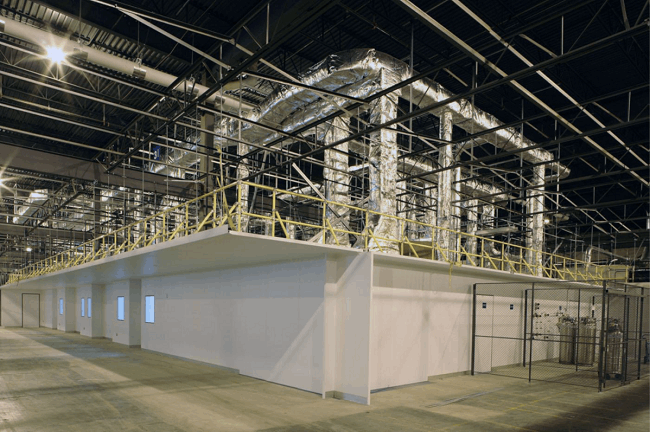
Image courtesy of G-CON Manufacturing
Podular
Podular systems are self-contained cleanrooms that are fabricated and validated off-site, including process equipment if needed. They are shipped complete to a site and then rigged into a shell space and connected to utilities (in an adjacent chase). These systems can be rapidly built and deployed — provided customization is minimized. These units can be constructed in 12 to 16 weeks. For some companies, these units provide financial flexibility, as they can be leased and depreciated like equipment. Overall, podular construction can reduce a typical 18-month construction project to 8 or 9 months. The swift delivery and high quality does come at a price, however, with the typical podular project costing more than $1000 per square foot. The figures below show the interior and exterior of podular build-outs.
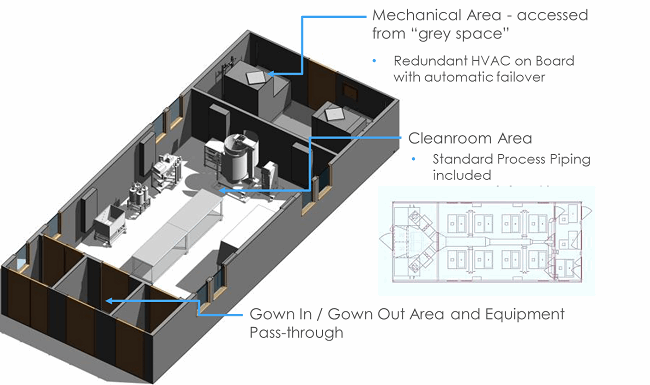
Images courtesy of G-CON Manufacturing
Construction Dos and Don’ts
As we conclude our series of articles, it is imperative to explain how designs are implemented in physical terms. Designs and specifications will have little meaning or provide little benefit if they are not implemented correctly in the field. As engineers and designers, we have a responsibility to “reach out and touch” our work. This last section provides some important best practices for directing the construction effort.
Walls
The days of using industrial gypsum board (epoxy coated) for a biological or an aseptic facility are long gone, and those who are still using such materials are creating a compliance and maintenance weak-point in their design. Any form of gypsum board creates and sheds dust during its lifecycle. In addition, all gypsum board (even the mold-resistant type) retains moisture, deteriorates under constant cleaning, and will proliferate mold.
The only acceptable wall systems for new construction are monolithic polymer wall systems. There are several different manufacturers with a wide spectrum of systems, from name brand systems like Trakion to simple FRP (fiber-reinforced polymer) panels. These modules and panels present a flat impervious and cleanable surface that will stand up to years of cleaning chemicals. For renovations, a polymer-based system called Mipolam can be used to covering and encapsulating aging walls, specifically in the cases where gypsum wallboard cannot be removed safely or without excessive generation of particulates and mold spores.
The keys to wall implementation are the skill of the installer, the erection of the wall sections, and the airtight closing/sealing of the seams. The fit and finish is critical to eliminate any external air infiltration.
In modern biotech facilities, the walls also provide the platform for many more fixtures than in legacy facilities — sprinkler heads, intercom speakers, fire/smoke alarms, cameras, facility alarms, and HVAC alarms are all mounted to them. These fixtures should not be mounted on the ceilings due to the potential for dirt, dust, and biological infiltration into the classified room via gravity.
Floors
Floors are technically the most vulnerable part of a facility, thanks to the wear and tear from personnel and equipment. In addition, thanks to gravity, it is the dirtiest part of the facility and the greatest source of plant-wide contamination. Floors within a classified area need to be made out of the mechanically hardest and toughest material, must be chemical resistant, and must not generate particles when impacted or stressed. There are several versions of hard polymer epoxy floors such as epoxy terrazzo (Stonhard), which have served the industry well for many years. The keys to installation include proper preparation of the substrate floor (time, temperature, composition, leveling), proper application layering, and buffing/polishing. Without a quality-oriented protocol, we have seen the following problems occur:
- Surface blooming, where the improper mixing of the top coating causes ring-like discoloration (i.e., blotches) and the appearance of a fine dust at those locations
- Rough sandpaper-like surfaces by the walls and/or ultra-slick surfaces in the center, caused by improper broadcast application, lack of cure time, or improper surface buffing. Floors should have a smooth feeling surface with an “orange peel” texture for footing.
- Cracking and splitting floors generally occur when the subsurface concrete floor is not prepared or is unstable. Rushing to get a floor installed without allowing the proper cure time can be potentially catastrophic for the entire site. We have seen an entire slab shift and open a 2-inch gap in the floor. Unfortunately, the owner exacerbated the situation by using hardware-store caulking to fill in the gap. A floor section patch can only be installed after settling, which requires an operational shutdown.
- We have seen an industrial paint that lasted only a few weeks after being applied to a concrete floor!
It is also important to understand what conditions and use the floor will be subjected to before selecting a floor system. If the floor not exposed to significant rolling equipment traffic, but it will see significant impact from steel pallets, dropped fitting, and/or vibrating equipment, even the hardest floor could chip or crack. In such cases, select a floor system with more elasticity, or “give”, such as nora rubber floors. These floor panels perform like the epoxy floors but have more resilience to impact.

Image courtesy of IPS-Integrated Project Services
In classified spaces, all floors need to be installed up the wall at least 6 inches and directly interface with the floor-level returns for the HVAC. The top floor section should have a polymer bead that seals it against and to the wall. This is for cleanliness and to protect the lower wall section from cleaning chemicals and wheeled equipment traffic.
All floor drains, traps, and clean-outs must not be installed in an EU grade A, B, or C space. If floor-level fixtures are needed in grade D or CNC space, they must be installed with a flat stainless steel cap with an O-ring seal, and care must be taken to minimize any gaps with the floor. Floor gaps and checkered brass covers are excellent sites for dirt to collect and microorganisms to breed.
Ceilings
Ceilings are often installed but not inspected properly during the construction phase of a project. Ceilings should only contain the HVAC supply and sealed lighting fixtures — no other penetrations. Ceilings must also be washed with the rotation of cleaning and disinfecting chemicals and therefore must be polymer-based monolithic material systems. A popular choice is a Gordon ceiling that integrates polymer panels with sealed tear-drop lighting fixtures and a sealed, gasketed interface to the HVAC. The ceiling panels are also sealed directly to the walls, thus providing an airtight envelope for the operating environment.
Like all other installations, the ceiling need to be visually and hand inspected, ensuring that:
- Gaskets are properly installed
- The HVAC register is anchored to the ceiling and does not modulate
- Double gasket seals are properly seated on top of observation windows
Image courtesy of IPS-Integrated Project Services
HVAC
In previous articles, we covered the key aspects of HVAC design. In our modern world of modular or podular building systems, the HVAC supply and return registers are physically fixed in position. In stick-built or renovation situations, the field installation must be by design and not by “the discretion of the installer.” The air flow patterns are critical and depend on the register locations. HVAC diffusers should be design-level selected and installed to provide coverage; however, many installers and “air balancers” will take great liberty with the adjustment of the diffusers and air supply to compensate for differential pressure requirements. Such compensation must be within the EU grade guidelines and must not disturb the air movement pattern within the room. More air is not necessarily better, as increasing overhead air velocity can backfire and force air to bounce off the floor and create a circulation pattern in the room that traps particulates and microorganisms. The careful inspection and adjustment of door seals and door sweeps, and the removal of return register gratings (if installed) and any obstructions, may be more effective.
Piping
Modern biotech facilities have a minimum of any piping. However, we need to mindful of stringing tubing and flex hoses overhead, creating a “jungle” of lines that are a haven for particulates and safety issues. The best approach is close proximity connections and wall-mounted hangers/chases for tubing, to keep them out of the way of the air flow and personnel traffic.
Conclusion
Newer polymer materials, prefabricated modules and sections, and Lego-like construction techniques will reduce construction dust, dirt, and inclusion of contaminants in modern bioprocessing facilities. These approaches are critical to minimizing construction “carry over” into the manufacturing areas during renovations — the most common cause of significant regulatory observations from the FDA.
Newer alternatives to constructing facilities can help manufacturers realize a major reduction in schedule and on-site costs. In addition, these emerging methods and construction materials can be significantly more compliant and easier to maintain long-term. In essence, they enable you to improve on time-to-market and regulatory compliance with facility maintainability as an added bonus. However, improvements in quality and schedule come with an increase in cost.
How do we balance such seemingly conflicting requirements? It comes down to the value of the product on the market and its early introduction and regulatory clearance. For biologics manufacturers, the result could be a rapidly deployed facility with a small footprint and a low operational cost — and a jump on the market. As we mentioned earlier, the schedule drives the technology, and cost can only be pushed so far in the face of quality requirements. For contract manufacturers (CMOs), it means a quicker tech transfer and an attractive facility that sells itself to prospective clients.
Throughout this series, we have articulated many trends, technologies, designs, and best practices for the design and construction of modern biological facilities. The ideas presented provide designers and builders many avenues to deliver a well-constructed and compliant facility. We also communicated the impact of cost, both initial investments and downstream improvements. The rest is up to the user and the engineer, but remember that there is no technology without investment, there is no compliance without quality, and you still get what you pay for. Cutting corners and pushing the envelope on cost and quality is only deceiving you and your management — and it will have its consequences.
This article origially appeared on BioProcess Online.



